Photo Gallery of Vegetable Problems
Brassicaceae
General Disease Management in Brassicas |
|||
Diseases Common to Brassica Vegetables |
|||
|
|||
Insects/Mites |
|||
|
|
|
||
Abiotic Problems Common to Brassica Vegetables |
|||
|
|
|||
Problems on Specific Brassicaceae Crops |
||
(Click on photo to enlarge)
General Disease Management in Brassicas
Production of Brassica Seed Crops in Washington State: A Case Study on the Complexities of Coexistence, Washington State University Extension Bulletin No. EM062E.
Crucifer Disease Guide - A Practical Guide for Seedsmen, Growers and Agricultural Advisors. Published by Seminis Vegetable Seeds, Inc.’s Plant Health Department and Seed Health Departments.
Small-Scale Cost-Effective Hot Water Seed Treatment
By Frank Morton (Wild Garden Seed), Tom Stearns (High Mowing Seeds), Nick
Andrews (OSU Small Farms Extension)
Hot water seed treatment is an important way of reducing the risk of seed-borne diseases, especially for organic Brassica growers (seed, fresh market or processed market) now that Pacific Northwest growers are dealing with black leg and potential light leaf spot epidemics. Hot water treatment can improve our defense against diseases like blackleg, light leaf spot, Verticillium, Fusarium, Xanthomonas, Alternaria, Botrytis and many seed-bourne viruses.
The authors have put together some slides with some practical ideas for
low-cost and efficient hot water treatment.
Download the Hot Water Treatment Slideshow.
Diseases Common to Brassica Vegetables
Disease: Black leg
Pathogens: Phoma lingam (sexual stage = Leptosphaeria maculans)
Host crops: Most members of the Brassicaceae (Cruciferae) = cabbage family, including broccoli, Brussels sprouts, cabbage, canola, cauliflower, various Chinese brassica vegetables, collard, kale, mizuna, mustard, oilseed rape, oilseed turnip rape, rutabaga, turnip, etc.), Sinapis (white and yellow mustard), and Raphanus (daikon and radish). Several wild species exist that may be infected by P. lingam including Descurainia (tansymustard), Sisymbrium (hedge mustard), and Thlaspi
(penny-cress). This is a quarantine disease in six counties in northwestern WA
and all counties east of the Cascade Mountains because of the risk of this
pathogen to the brassica vegetable seed industry.
On-Line Resources:
Video: Blackleg Disease and Resistance Management. Published by the Canola Council of Canada.
Pacific Northwest Plant Disease Management Handbook: Seed Crop, Crucifers (Brassica and Raphanus spp.)-Black Leg
Black leg in Brassicaceae crops and wild crucifers: 2014 outbreak in the Willamette Valley of Oregon
Black Leg, Light Leaf Spot, and White Leaf Spot, Cynthia Ocamb, PhD., Plant Pathologist, OSU Extension, Associate Professor--Botany & Plant Pathology.
Fungicides for Control of Black Leg, David Priebe, Pesticides Program, Oregon Department of Agriculture.
Crucifer Seed Emergency Rule
Effective July 16, 2015
For those of you who work with crucifer crops of any kind (oilseed, cover, processing, fresh market, seed, forage, etc.), here is important and time-sensitive information from Victor Shaul of the WSDA Seed Program on proposed amendments to a relavant quarantine rule in WA.
"The public hearing for the Crucifer seed quarantine was held on July 7th. There were some substantive changes that occurred during the hearing. The main change; in red below, was at the request of those in attendance at the hearing. In essence, this change says that seed that was produced within the regulated areas (both the Eastern and Western Washington areas) to be planted in the Eastern Washington regulated area does not have to be treated. In converse, seed originating elsewhere will need to be treated.
"To recap the changes the rule proposal will:
· Not change any requirements already in place for Crucifer seed to be planted in Western Washington.
· Will include Eastern Washington under the Crucifer Quarantine rules.
· Will require any Crucifer seed that will be planted in Eastern Washington to be laboratory tested and found free of Blackleg.
· Will require any Crucifer seed from an origin outside the regulated areas* that will be planted in Eastern Washington to be treated – various options.
· Will require any Crucifer seed that will be planted in Eastern Washington to have each container tagged with a Crucifer Quarantine tag issued by the Department.
*For reference an area outside the regulated areas would be any area outside the Western Washington counties of Clallam, Island, Lewis, Skagit and Snohomish and all counties in Eastern Washington.
"The second major occurrence was the request to implement the rule changes immediately. The reasoning behind this request was the time it would take with the normal rule process for the rule changes to become effective. In essence it would miss the fall planting season. It was felt that this is such an important threat to Crucifer production that an emergency rule should be enacted as soon as possible. To that end the Director signed the emergency rule effective on Thursday July 16th. As of that date all Crucifer seed to be planted in Eastern Washington must be quarantine compliant.
"Lastly, due to the requested changes in the language there will be a second public hearing. Comments can be sent by e-mail if that is more convenient. I will send that information as well.
"I would ask everyone to assist in spreading the word about this through whatever means you can. We are currently working on a fact sheet and mailer and hope to have that completed soon with plans to send to as wide of an audience as possible.
"Additionally, you will see attached a Crucifer Quarantine tag request form. Please note this is specific for seed that is to be planted in Eastern Washington. Please pass along to whomever in your company that will be handling this.
"I have also been asked what kind of seed treatments can be used for Blackleg. Here is a resource you can use: https://cru66.cahe.wsu.edu/LabelTolerance.html and of course consult your seed treatment/chemical provider for further assistance."
Please provide feedback or recommendations to:
Victor Shaul, WSDA Seed Program Manager
vshaul@agr.wa.gov
Further Info:
- Proposed Crucifer Quarantine Rule Amendments (May, 2015)
- Proposed Rule Amendments, Amendatory Section (July, 2015)
- Crucifer Quarantine tag request form (.docx)
Addressing Blackleg in the Willamette Valley: Oregon Department of Agriculture permanent ruling released on black leg of brassicaceae in January 2015 – see the Brassica Production Districts document, and the OSDA Permanent Ruling document titled ‘Crucifer blackleg disease requirements moved into one regulation; removes same requirements from rapeseed production districts,’ below.
- Oregon Secretary of State Certificate and Order for Filing - PERMANENT ADMINISTRATIVE RULES: Crucifer blackleg disease requirements moved into one regulation; removes same requirements from rapeseed production districts.
- Brassicaceae Production Districts and Rapeseed Control Areas (603-052-0860), Oregon Department of Agriculture.
Management of Black Leg in Oregon on Brassica seed crops, a Clinic Close-up, Oregon State University Extension Service.
Management of Black Leg in Oregon on Vegetable Brassica Crops and Seed Crops, a Clinic Close-up, Oregon State University Extension Service.
Disease: Black rot
Pathogens: Xanthomonas campestris pv. campestris
Host crops: Most members of the Brassicaceae (Cruciferae) = cabbage family, including
broccoli, Brussels sprouts,
cabbage, canola,
cauliflower, various Chinese brassica vegetables, collard, kale, mizuna,
mustard, oilseed rape, oilseed turnip rape, rutabaga, turnip, etc.), Sinapis (white and yellow mustard), and Raphanus (daikon and
radish). Most
wild species can be infected by this pathogen. This is a quarantine disease in parts of six counties in northwestern Washington because of the risk of this pathogen to the brassica vegetable seed industry.
Online Resources:
Cabbage and Cauliflower (Brassica sp.)-Black Rot, Pacific Northwest
Handbooks, a Pacific Northwest Extension Publication.
Black Rot of Crucifers, Fact Sheet, Cooperative Extension, New York State,
Cornell University.
Field Scouting Guide: Black Rot of Brassicas, Growing Produce, Meister Media
Worldwide’s Horticulture Group.
Managing Black Rot of Cabbage and other Crucifer Crops in Organic Farming
Systems, eOrganic, eXtension Foundation (extension.org).
Brassicas, Black Rot, UMass Extension vegetable Program Fact Sheet, Center
for Agriculture, Food and the Environment, College of Natural Sciences,
University of Massachusetts, Amherst, MA.
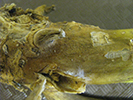
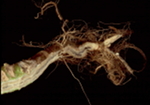
Disease: Clubroot
Pathogen: Plasmodiophora brassicae
Host crops: Broccoli, cabbage, cauliflower, brassicaceae (cruciferous) weeds, and radish.
 |
 |
 |
| Stunting from clubroot. | Below-ground symptoms of clubroot. | |
| Photo Source: Lindsey du Toit | ||
On-Line Resources:
Pacific Northwest Plant Disease Management Handbook: Cabbage and Cauliflower (Brassica sp.)-Clubroot
Clubroot of Crucifers. Vegetable MD Online.
Clubroot. Wikipedia.
Clubroot of vegetable brassicas – towards integrated control. New Zealand Institute for Crop & Food Research Ltd.
Clubroot of Crucifers. The Ohio State University Extension.
Managing Clubroot: Equipment Sanitation Guide. Canola Council of Canada
Top 10 tips from the 2013 International Clubroot Workshop. Canola Watch, Canola Council of Canada.
See Diseases, pests, and other problems common to many vegetables: Clubroot of brassica vegetables.
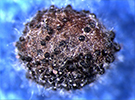
Disease: Downy mildew
Pathogens: Hyaloperonospora parasitica = Peronospora parasitica
Host crops: Most members of the Brassicaceae (Cruciferae) = cabbage family, including cabbage and cauliflower.
 |
 |
 |
 |
| Downy mildew sporulation on cabbage leaves. | Sporulation of downy mildew on a cabbage seed pod in a cabbage seed crop. | ||
| Photo Source: Lindsey du Toit | |||
Online Resources:
Downy Mildew of Crucifers. Plant Pathology Fact Sheet. Florida Cooperative Extension Service.
Brassica Downy Mildew. University of Massachusetts Amherst.
Disease: Light leaf spot
Pathogens: Cylindrosporium concentricum (sexual stage: Pyrenopeziza brassicae)
Host crops: Light leaf spot has been observed causing disease in canola (oilseed rape can be very susceptible), forage Brassica species, “field” turnip, other Brassica members including wild mustard, volunteer black mustard, vegetable Brassica seed fields, and Brassica species used as cover crops. It is likely that all brassicas crops grown in the Pacific Northwest are susceptible with a range of susceptibility within each crop species.
Online Resources:
Light leaf spot OSU Disease Alert in crucifers 17 June, 2014 (pdf)
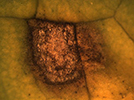
Disease: Ring spot
Pathogen: Mycosphaerella brassicicola
 |
 |
Photo Source: D.A. Inglis |
|
On-Line Resources:
Disease: White leaf spot
Pathogens: Pseudocercosporella capsellae (sexual stage: Mycosphaerella capsellae)
Host crops: White leaf spot and gray stem were observed during 2014 in canola research fields as well as in commercial seed fields of forage Brassicas and “field” turnip. White leaf spot was also detected in volunteer black mustard and forage fields. Susceptible hosts reportedly include species of Brassica (broccoli, cabbage, canola, cauliflower, Chinese cabbage, mustard, turnip, etc.) as well as radish and horseradish. Weedy types such as wild radish, wild mustard, and shepherd's purse are susceptible to white leaf spot and gray stem.
Online Resources:
White leaf spot OSU Disease Alert in Brassiceae (pdf)
Disease: White mold
Pathogens: Sclerotinia sclerotiorum
Host crops: Most members of the Brassicaceae (Cruciferae = cabbage family) and many other vegetables (see Diseases, pests, and other problems common to many vegetables: White mold )
 |
 |
 |
 |
| White mold symptoms on a cabbage head. | Severe white mold in an overwintering cabbage seed crop. | Stem canker in a brassica seed crop caused by Sclerotinia sclerotiorum. | |
| Photo Source: Lindsey du Toit | |||
Online Resources:
Management of Sclerotinia in Turnip Seed Crops - A Clinic Close-up, Oregon State University Extension Service
See Diseases, pests, and other problems common to many vegetables: White mold.
Insects/Mites
Common name: Root maggots (cabbage maggot and seedcorn maggot)
Latin binomial: Delia brassicae = Delia radicum (cabbage maggot), and Delia platura (seedcorn maggot)
Host crops: Cabbage maggot can damage and destroy root systems of all cole (crucifer or Brassicaceae) crops. Tunnels from the feeding maggots can become numerous in roots in crops with severe infestations. The tunnels provide wound sites for pathogens, particularly bacteria that can cause bacterial soft rot. The seedcorn maggot can affect many vegetable crops including snap, kidney, and lima
beans,
onion, corn, turnip, pea, cabbage, and cucurbits. They cause the most damage in spring to newly emerging seedlings, and can cause severe losses in plant stand.
Online Resources:
PNW Insect Management Handbook section on cabbage maggot affecting radish
Abiotic Problems Common to Brassica Vegetables
Problem: Boron (B) deficiency
Crops affected: Most crops can develop symptoms of boron (B) deficiency. Brassica or cole crops have moderate to high B requirements. B deficient cole crops can develop cracked, corky stems, as well as petioles and midribs. Broccoli, cabbage and cauliflower stems may become hollow and discolored. Cauliflower curds may turn brown and leaves roll and curl. Cabbage heads may be smaller than normal and discolored yellow. Cauliflower is the most sensitive of cole crops to B deficiency.
Online Resources:
Boron Deficiencies in Cole Crops, University of Delaware Extension
https://customers.hbci.com/~wenonah/min-def/cauliflr.htm
https://www.ipmimages.org/browse/subimages.cfm?sub=18132
https://www.spectrumanalytic.com/support/library/ff/B_Basics.htm
Boron mobility in plants. Chapter 7 from the book Plant and Soil by Patrick H. Brown, Department of Pomology, University of California, Davis and Barry J. Shelp, Department of Horticultural
Science, University of Guelph, Guelph, Ontario.
Boron Deficiency Symptoms U.S. Borax Corp.
Boron in vegetables U.S. Borax Corp.
Problem: Edema
Cause: A physiological problem prominent when air is cooler than the soil, soil moisture is high, and relative humidity is high. The low plant transpiration rates combined with an increase in water absorption by roots from the soil leads to increased cell turgor pressure, resulting in eruption of epidermal cells as the inner cells enlarge. Protrusion of the inner cells causes epidermal cells to die and discolor, resulting in a ’warty’ appearance that can be misidentified as a disease. Symptoms are usually worse on the lower leaf surface and on older (lower) leaves.
Host Crops: Numerous vegetables including spinach, brassicas, tomato, etc. Vegetables with waxy leaves, e.g., brassicas, tend to be most susceptible.
Photo Source: Pop Vriend Seed Co.
-thumb.jpg) |
| Symptoms of edema on the lower (abaxial) surface of a cabbage leaf, including calloused/warty protruberances from bursting of epidermal cells. |
Online Resources:
Pacific Northwest Handbooks: Cabbage and Cauliflower Brassica SP Oedema Edema
What are these bumps on my vegetables? Edema or oedema: It doesn’t matter how you spell it, it still doesn’t look good. What is it, what causes it and how can I prevent it? Michigan State University Extension
Problem: Redheart
Cause: Freeze damage to inner leaves of a brassica head as a result of an extended period (>24 hours) of freezing in the field or in storage. Damage is often irreversible. Leaves several layers inside the head become watersoaked/glassy while outer leaves of the head appear normal. Internal affected leaves become tan or red in color, and may dry to a papery texture. A bad odor may develop. A dark zone may delimit the affected vs. healthy areas. Similar symptoms can be caused by exposure of cabbage heads to low oxygen levels or high carbon dioxide levels in controlled atmosphere storage.
Crops affected: Brassica vegetable crops with heads, e.g., cabbage. Some cultivars are more sensitive to redheart than others.
 |
 |
| Photo Source: Osborne International Seed Co. |
|
Harvest crops before extended periods of severe frost. If freezing conditions occur, allow heads to thaw completely before they are harvested. Monitor the heads at harvest for glassiness of the internal leaves. For longer-term storage, only store cabbage heads that have not been exposed to frost. Ventilate storage rooms adequately at temperatures just above freezing and high humidity.
Online Resources:
University of California Postharvest Technology. Cabbage (Round and Chinese types): Recommendations for Maintaining Postharvest Quality
Problem: Stem splitting
Crops affected: Any brassica crop grown for seed can develop stem splitting under conditions that promote very rapid growth (high soil moisture and warm temperatures).
Online Resources:
Our pages provide links to external sites for the convenience of users. WSU Extension does not manage these external sites, nor does Extension review, control, or take responsibility for the content of these sites. These external sites do not implicitly or explicitly represent official positions and policies of WSU Extension.