Cherry Bark Tortrix: Biology and Population Management, 2002
L. K. Tanigoshi and Todd A. Murray
Washington State University
Vancouver Research and Extension Unit
Vancouver, WA 98665-9752
tanigosh@wsu.edu, tamurray@coopext.cahe.wsu.edu
Introduction
 |
|
Figure 1. Adult cherry bark tortrix moth.
(Photo courtesy E. LaGasa) |
Cherry bark tortrix (CBT), Enarmonia formosana (Scopoli) was first reported in 1989 infesting cherry trees in Richmond, British Columbia. This species has been known from the European literature since 1837. CBT (Figure 1) is classified in Tortricidae, a large family of small, broad-winged moths containing over 1200 species in North America. The larvae of tortricids feed on a large array of perennial plants, commonly in rolled or tied leaves, thus are called leafrollers. However, cherry bark tortrix larvae feed within the bark and are bark borers. High infestation densities of CBT will cause girdling and eventual death of cherry trees. Secondarily, CBT predisposes trees to diseases, insects, and freezing mortality factors.
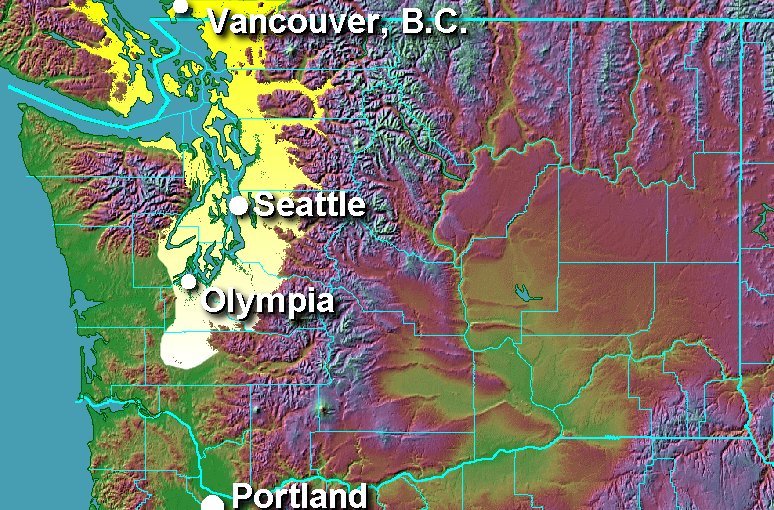 |
| Figure 2. Current distribution of CBT in the Pacific Northwest of North America. |
Internationally CBT is known to occur in Eurasia, Africa and North America.It was first detected in 1991 by the Washington State Department of Agriculture in the Peace Arch State Park, near Blaine, Whatcom County.
In 1996, a survey of randomly selected cherry trees in Bellingham found 75-80% of them were infested with CBT. Their populations are well established in the humid, temperate, broadleaf forest regions of western lowland valleys in the Cascade Mountain Range along Puget Sound. Cherry bark tortrix has been moving southward in steady surges with the most southern trap site in 1998 near Centralia, about 35 miles north of the Columbia River and Oregon State border. In 2000, two moths were trapped in Portland. The 2002 trapping season has confirmed CBT’s establishment in the city of Portland (Figure 2).
Biology
 |
| Figure 3. Black bars (circled in blue) are characteristic of adult CBT. (Photo courtesy E. LaGasa) |
The forewing of CBT measures from 0.6 to 0.7 inches and is cryptically ornate with a dark brown to black-purplish sheen appearance, yellow-orange markings and several white patches along the leading edge of the forewings. Black bands are characteristic markings of CBT (Figure 3). Females emerge about two weeks after male flight in May and begin laying eggs 1-4 days after emergence. Eggs measure 0.03 inches in diameter, slightly domed-shaped and turn a milky white to salmon pink color.
 |
|
Figure 4. Cherry trunk showing heavy frass accumulation and excess gummosis.
|
Eggs are commonly laid singly or in small patches near previously infested bark marked with lesions, gummosis, and frass mixed with silken webbing (Figure 4). Larvae mature through 5 separate instars and are 0.3 to 0.4 inches long when fully grown. Color of larvae range from light brown to darkish pink with a light brown head. CBT has one generation a year and adult flight period extends from May to September. The specific life stages of CBT have no synchronous pattern producing this long flight period (Figure 5).
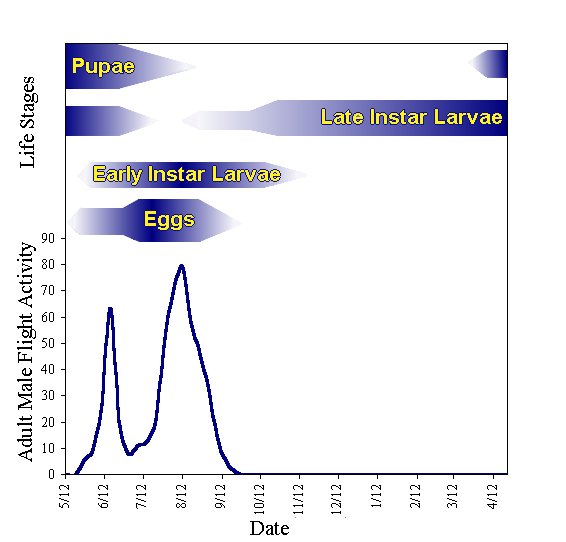 |
| Figure 5. Typical phenology of CBT. Although it appears to have two generations, there is only one. |
Cherry bark tortrix overwinter as a mixed stage of 2nd to 5th instar larvae. They will feed at temperatures above freezing throughout the winter months. Late instar larvae are fully grown by the following spring. They then pupate within their silken cocoon which is commonly formed within the externally formed “frass tubes” (Figure 6). This population emerges about 15 days later as adults in late April/early May, which corresponds to the first peak of flight activity in June. The remaining population produces a peak of adult activity in early August (Figure 5). Having two periods of flight activity is referred to as a “bimodal” pattern and is consistent with observations of CBT in Europe.
 |
| Figure 6. Frass tube constructed by the larvae of CBT. (Photo courtesy E. LaGasa) |
First instar larvae feed on the bark and outer sapwood while the 2nd through 5th instars make tunnels between the bark and cambium (Figure 7), but do not penetrate the hard wood. Infestations usually occur upward from the base of the tree. Infestations are easily recognized by reddish-orange colored frass accumulations or frass tubes near gallery entrances.
 |
| Figure 7. View of CBT larval gallery with the outer bark removed. |
Larvae damage trees by direct feeding which reduces or excludes transportation of nutrients to the roots. The feeding causes exudation of gum and deformation of bark growth on the main branches and trunk. Along infested sides of the tree, dieback of new and old growth can occur from the portion girdled (Figure 8). Indirect damage occurs through the formation of habitats for secondary pests such as bark beetles, fungi, and increasing susceptibility of infested trees to successive years of freezing damage which can result in thedeath of the tree.
 |
| Figure 8. Canopy die-back due to high infestations of CBT. |
Host Range
| Table 1. Species and generic checklist of identified hosts of CBT in Washington. Denotes the first known host record for CBT. |
|
| Species Name | Common Name |
| Prunus | Cherry, Plum, Peach, Apricot |
| P. emarginata | Bitter Cherry (Native, U.S.) |
| P. avium | Sweet Cherry |
| P. cerasus | Sour Cherry |
| P. serrulata | Oriental Flowering Cherry |
| P. serrula | Chinese Flowering Cherry |
| P. yedoensis | Japanese Flowering Cherry |
| P. incisa | Fuji Cherry |
| P. sargentii | Sargent Cherry |
| P. subhirtella | Weeping Flowering Cherry |
| P. cerasifera | Flowering Plum |
| P. domestica | Fruiting Plum |
| P. armeniaca | Apricot |
| P. persica | Peach |
| P. lusitanica | Portuguese Laural |
| Malus | Apple/Crab Apple |
| M. oregonensis | Crab Apple (Native, U.S.) |
| M. pumila | Fruiting Apple |
| Pyrus | Pear |
| P. communis | Fruiting Pear |
| Crataegus* | Hawthorn |
| C. douglasii* | Black Hawthorn (Native, U.S.) |
| C. monogyna* | Single Seed Hawthorn |
| Sorbus | Mountain Ash |
| Cydonia | Quince |
| Pyracantha | Firethorn |
| Photinia* | |
| P. xfrasero* | Fraser Photina |
All rosaceous wood shrubs or trees are susceptible to attack by CBT. Since the establishment of cherry bark tortrix, it has expanded its host range to all Prunus, Malus, Crataegus, Pyrus, Pyracantha, Cydonia, Sorbus and Photinia species. For a checklist of confirmed hosts of CBT, see Table 1. Prunus trees are most preferred of all genera; there is a hierarchy of preference between Prunus species and even between varieties. However, once CBT is established in an area, host switching becomes rapid as preferred hosts are depleted.
 CBT was characterized as preferring mature and older trees in the European literature. This is not the case in North America. CBT is found on many different age classes and size classes of hosts, even on suckers of fruit trees. CBT is an opportunistic pest and selects the host that is most accessible. The preferred habitats of CBT all have one thing in common, easy access to the innerbark. Typical infestation sites include graft unions, branch unions, mechanical wounds, cankers, poor pruning cuts, any site with textured splits in the bark (Figure 9). Tree selection, tree health and good maintenance practices will decrease host susceptibility to CBT infestation.
CBT was characterized as preferring mature and older trees in the European literature. This is not the case in North America. CBT is found on many different age classes and size classes of hosts, even on suckers of fruit trees. CBT is an opportunistic pest and selects the host that is most accessible. The preferred habitats of CBT all have one thing in common, easy access to the innerbark. Typical infestation sites include graft unions, branch unions, mechanical wounds, cankers, poor pruning cuts, any site with textured splits in the bark (Figure 9). Tree selection, tree health and good maintenance practices will decrease host susceptibility to CBT infestation.
Control Strategies
Cherry bark tortrix is widely established on naturalized cherries in untreatable natural landscapes and is threatening native species such as bitter cherry, crabapple and black hawthorn. Various survey techniques were used in 1997 and 1998 to identify and quantify the impact of native natural enemies of the CBT. Total parasitism in the field was 1.7% in 1997 and 2.l% in 1998. Despite these low levels of parasitism, we recovered at least one species from each of six different parasitic wasp families (Figure 10). It is clear that currently no natural enemy or complex endemic to the Pacific Northwest is offering significant control of CBT populations.
 |
| Figure 10. Ichneumonid parasitoid responsible for most parasitization in Washington State. |
According to the world literature, little attention has been paid to the European natural enemies of CBT. From this fact, coupled with our native natural enemy survey, we recognize that exploration and importation of exotic parasitoids (i.e., classical biological control), native to the homeland of the CBT is the most rational and economic approach to bringing CBT into ecological balance.
 |
| Figure 11. Dr. Lynell Tanigoshi dissecting a cherry tree in search of CBT natural enemies in Switzerland. |
We have conducted two intensive field surveys in different sweet cherry regions of Germany and Switzerland in collaboration with CABI Bioscience, Delemont, Switzerland, during mid-summer of 1998 and 1999 (Figure 11). About 60 locations were intensively surveyed and frass tubes of CBT were found in all locations. As hoped for, non-economic CBT densities were found throughout the sweet cherry region surveyed. This strongly suggests that CBT is regulated at low economic levels by its co-evolved complex of natural enemies. Five different genera of the parasitic wasp family Ichneumonidae were obtained in the Rhine River Valley on commercial sweet cherry in Germany (Table 2).
Table 2. Ichneumonid species reared from larvae and pupae of CBT. |
|
| Species | Locality |
| Campoplex dubitator | Bamlach, Germany |
| Campoplex dubitator | Weil am Rhine, Germany |
| Campoplex dubitator | Oedsbach bei, Offenburg, Germany |
| Liotryphon crassiseta | Oedsbach bei, Offenburg, Germany |
| Isadelphus sp. | Oedsbach bei, Offenburg, Germany |
| Campoplex dubitator | Dossenheim, Germany |
| Alomyini (Phaeogenini) | Dossenheim, Germany |
| Pimpla sp. | Dosenheim, Germany |
| Pimpla hesperus | Bellingham, WA |
| Pimpla hesperus | Seattle, WA |
| Itoplectis quadriangulatus | Bellingham, WA |
| Itoplectis quadriangulatus | Seattle, WA |
The importation and release of exotic natural enemies of arthropod pests has proven to be cost effective and safe. Safety comes from proper screening and testing of the target organism prior to field release to meet federal regulations pertaining to the import of exotic arthropod natural enemies for field release. All aspects of quarantine, importation and augmentation of potential exotic natural enemies will be coordinated directly through the Department of Entomology, WSU’s recent USDA/APHIS certified Northwest Biocontrol Insectary and Quarantine (NWBIQ) facility (Figure 12). Field established native and exotic parasitic natural enemies require little management and they minimize the expense and hazards of pesticide applications.
 |
| Figure 12. Terry Miller preparing imported CBT larvae at WSU’s Northwest Biocontrol Insectary and Quarantine facility. |
Cherry bark tortrix poses the most immediate threat to our urban landscapes west of the Cascade Mountains. Vancouver, British Columbia has already experienced the loss of priceless, historically important trees that will forever change the landscape of the region. Secondly, there is a concomitant increase in costs to remove tree prematurely and to replant with non-host plants. Currently tree removal is the only treatment available when trees are declining. The City of Seattle has just begun to realize these repercussions in its landscapes that are generously interplanted with CBT susceptible rosaceous hosts. Once CBT becomes established in a nursery, orchard or ornamental landscape, there will be continuous management costs year after year to reduce CBT’s potential for long term damage. In addition to this direct damage, indirect costs of quarantines to infested states
will impose further economic damage to the ornamental industries of Washington State and British Columbia. Moreover, CBT is slowly moving in surges southward toward the Columbia River and Oregon State border.
The preliminary studies summarized above represent the starting point toward understanding the current status of CBT in North America and the potential impact of an associated native complex of parasitoids. Ultimately, it is recognized that the importation of new parasitoids, native to the presumed homeland of CBT, is the most rational and economic approach for reducing CBT below economic levels if natural biological control cannot be attained in Washington State.
For control recommendations, please see the Pacific Northwest Insect Control Handbook, Bulletin PLS-67 and consult your local Extension agent.